-
PDF
- Split View
-
Views
-
Cite
Cite
Outi Savolainen, Sonja T. Kujala, Catherina Sokol, Tanja Pyhäjärvi, Komlan Avia, Timo Knürr, Katri Kärkkäinen, Sheila Hicks, Adaptive Potential of Northernmost Tree Populations to Climate Change, with Emphasis on Scots Pine (Pinus sylvestris L.), Journal of Heredity, Volume 102, Issue 5, September-October 2011, Pages 526–536, https://doi.org/10.1093/jhered/esr056
Close - Share Icon Share
Abstract
The adaptive potential of the northernmost Pinus sylvestris L. (and other northern tree) populations is considered by examining first the current patterns of quantitative genetic adaptive traits, which show high population differentiation and clines. We then consider the postglacial history of the populations using both paleobiological and genetic data. The current patterns of diversity at nuclear genes suggest that the traces of admixture are mostly visible in mitochondrial DNA variation patterns. There is little evidence of increased diversity due to admixture between an eastern and western colonization lineage, but no signal of reduced diversity (due to sequential bottlenecks) either. Quantitative trait variation in the north is not associated with the colonizing lineages. The current clines arose rapidly and may be based on standing genetic variation. The initial phenotypic response of Scots pine in the north is predicted to be increased survival and growth. The genetic responses are examined based on quantitative genetic predictions of sustained selection response and compared with earlier simulation results that have aimed at more ecological realism. The phenotypic responses of increased growth and survival reduce the opportunity for selection and delay the evolutionary responses. The lengthening of the thermal growing period also causes selection on the critical photoperiod in the different populations. Future studies should aim at including multiple ecological and genetic factors in evaluating potential responses.
In Europe, northernmost animal and plant populations have colonized their current distribution areas during the last 10 000 years. In this short time span, they have become genetically differentiated from the Central European and other more southern populations (e.g., Lagercrantz and Ryman 1990; van Rossum and Prentice 2004). Several evolutionary factors may have led to this genetic differentiation (Pamilo and Savolainen 1999), but reciprocal transplant experiments have shown that many populations have become adapted to the local conditions due to differential selection (Turesson 1922; Olsson and Ågren 2002; Savolainen et al. 2007; Leinonen et al. 2009).
Local adaptation and range expansion at the species margin depend on an interplay between selection and gene flow (Kirkpatrick and Barton 1997; Lenormand 2002). On the one hand, gene flow provides raw material for evolution, and on the other hand, extensive gene flow may prevent adaptation at species margins (Haldane 1956; García-Ramos and Kirkpatrick 1997). When there are no direct physical barriers, interspecific interactions can also have an especially important role in limiting adaptation and range expansion (Case et al. 2005).
Despite the importance of local adaptation, predictions on species composition often proceed without taking genetics into account, assuming that climatic envelopes for the species are maintained (IPCC 2001; Kellomäki et al. 2001; Thomas et al. 2004). It seems important to include genetic aspects because evolutionary change has also accompanied historical range shifts (Davis and Shaw 2001).
Understanding the mechanisms of previous adaptation to new climates by migration and evolution and knowledge of the current state of the patterns of genetic variation provide the possibility to also examine the potential for future adaptation (Petit et al. 2008). The role of migration and evolutionary responses of trees to climate change at large have been considered earlier (Davis and Shaw 2001; Saxe et al. 2001; Savolainen et al. 2004; Aitken et al. 2008). Petit and Hampe (2005) have emphasized the importance of responses at the southern rear edge. Here we examine the adaptive potential of northernmost tree populations to changing climate, often using Scots pine (Pinus sylvestris L.) as the specific example. We first describe the present patterns of genetic variation, next consider the history of migration and evolution that led to the current patterns, and last consider the potential for future changes. The evaluation is not only based partly on our own recent work but also on the extensive forestry research on trees in Scandinavia and Finland.
Materials and Methods
We have earlier described sequence variation in a limited set of genes and populations (Pyhäjärvi et al. 2007). Phenotypic variation in timing of budset has also been studied earlier (García-Gil et al. 2003; Notivol et al. 2007). The sequence and phenotypic data added here have been obtained using methods described in these articles. Here we deal with samples from 3 populations, Norra Gullabo, Sweden (56°28', 15°55'), Kolari, Finland (lat 67°11', long 24°03'), and Usinsk, Russia (66°05', 57°30'). The sample sizes from each of these populations were 10 haploid (megagametophyte) samples per locus. The set of 10 loci, candidate genes for cold tolerance and budset, is described in more detail in elsewhere (Pyhäjärvi et al. 2007; Wachowiak et al. 2009; Kujala ST, Savolainen O, in preparation) The loci and the number of nucleotides sequenced were as follows: from Pyhäjärvi et al. (2007) and Kujala ST, Savolainen O (in preparation) col1 (constans like 1, length 3847), gi (gigantea, fragment 1, 402, fragment 2, 1370), phyn (phytochrome N, 6683), lp2 (s-adenosyl methionine synthetase, 1185); from Wachowiak et al. (2009)dhn1 (dehydrin 1 1357); and from Kujala ST, Savolainen O (in preparation) cry1 (cryptochrome 1, 4762), ft4 (flowering locus T 4, 2545), prr1 (pseudo-response regulator 1, 4183), ztl (zeitlupe, 1182), and myb (myb-related CCA1-like, clade II, 3113) .
Here we use these loci to compare diversity of populations and describe their divergence. Earlier work has shown that, in general, signals of selection at these loci are not strong, and a set of numerous loci will have overall patterns similar to loci presumed to be neutral (Pyhäjärvi et al. 2007; Wachowiak et al. 2009). The analyses are based on 10 haploid megagametophytes per locus. We also use records of pollen transport and deposition to look at the quantities of pollen available, its potential source, and the timing of dispersal. Methods for monitoring pollen deposition are given in Hicks (2001) and for the timing of pollen dispersal in Sokol C, Pessi AM, Huusko A, Hicks S, Kubin E, Heino S (in preparation).
Current Patterns of Genetic Variation and Adaptation
Scots pine has the widest distribution range of all pine species and populations are found in a wide range of environmental conditions, extending from Scotland to Eastern China and from southern Spain to northern Scandinavia and Finland. There is no physical barrier at the northern range limit, and selection imposed by the environmental conditions may determine the range in the north.
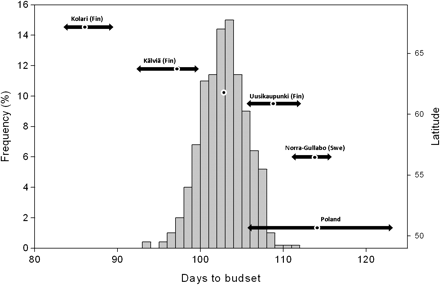
An essential aspect of climatic adaptation is the synchronizing of growth and reproduction with favorable seasons. Extensive studies have documented variation between populations in traits related to the timing of growth and pollen shedding or female strobilus maturity in Scots pine (Heikinheimo 1949; Eriksson et al. 1980; Chung 1981; Mikola 1982; Aho 1994; Oleksyn et al. 1998; García-Gil et al. 2003; Andersson and Fedorkov 2004; Notivol et al. 2007), whereas studies of the nuclear genome neutral markers have shown very low differentiation between populations within the continuous range of P. sylvestris (Gullberg et al. 1985; Pyhäjärvi et al. 2007). Figure 1 shows an example of variation of family means in the timing of setting the terminal bud in first-year seedlings. The southern Finnish population has much within-population variation for the timing of budset but is highly differentiated from other populations. For the other populations, the population means (and the ranges of family means) are shown. Interestingly, the differentiation is higher between southern Finland and the north than southern Finland and the south; for 5-degree latitude difference to the south, the mean budset difference between populations is 11 days, to the north, the difference is 17 days. The cline is thus at its steepest in the north. Many other species of conifers have similar extensive clines, as for instance Sitka spruce (Picea sitchensis) in Canada (Mimura and Aitken 2007; Mimura and Aitken 2010).
Histogram of family means of timing of budset in first-year seedlings of Scots pine (Pinus sylvestris) in a southern Finnish population (Punkaharju) compared with means (dots) and ranges (horizontal arrows) of other populations. Seeds were germinated in early June and grown in a common garden experiment in a greenhouse with close to ambient temperature and day length in 2008 at Haapastensyrjä (Southern Finland). The latitude of origins of populations is indicated as the position relative to right y axis. For locations of the populations, see Figure 2.
This pattern of quantitative trait variation is consistent with evidence of local adaptation showing that native populations perform better than transferred ones (Savolainen et al. 2007). Southern populations transferred to the north suffer increased mortality. Northern populations grow and survive better in more southern conditions but do not outperform the local southern populations. The pattern of local adaptation holds for most of the range from southern Sweden northward, but right at the northern range the pattern seems to break down (Savolainen et al. 2007). Northern populations of Scots pine and many other tree species are limited by cold temperatures (Eriksson et al. 1980; Rehfeldt et al. 2002; Reich and Oleksyn 2008). A lack of ability to adapt to the more northern conditions may have helped to shape the current northern edge of the distribution range.
Colonization of the North
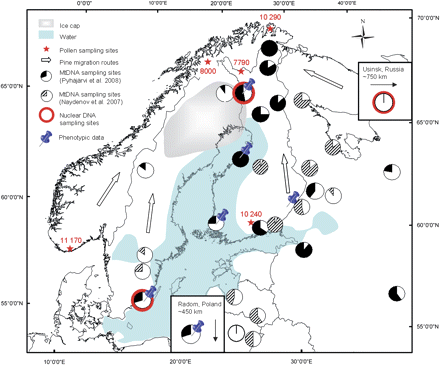
During the ice age, pine populations were found in the Iberian and the Italian peninsulas as well as in the Balkans (Cheddadi et al. 2006), but fossil evidence suggests that populations were probably also found north of these areas in Central Europe (Willis and van Andel 2004). It is known from the pollen assemblages preserved in lake sediments that in Fennoscandia, when the ice of the last ice age began melting, the newly revealed land along the retreating ice margin was first colonized by a pioneer vegetation of herbs and dwarf shrubs. These were quickly followed by tree birches, and it was into this birch-dominated landscape that Scots pine spread (Donner 2005). Scots pine is able to grow on a wide range of substrates and eventually outshades birch so that it was able to occupy the majority of the areas formerly covered only by birch and also to form extensive forests. Around 10 000 years ago, the last remnant of the ice sheet was situated over northern Sweden and, to the east of it, there was an extensive body of water, the Ancylus lake (Figure 2). Together they formed an effective barrier to migration, especially to northern Sweden. Migration was possible only via the land areas of Finland to the east of the Ancylus lake or along the narrow coastal strip of Norway, which had become ice free at an early stage in the deglaciation. Similar early ice-free areas also existed in the far north of Norway, Finland, and Russia, and there is some evidence to suggest that Scots pine was sparsely present in this area more than 10 000 years ago (Seppä 1996). On the basis of the available fossil pollen data, therefore, different parallel postglacial migration routes for Scots pine can be hypothesized: from southern Fennoscandia/Karelia northward on the eastern side of the Bothnian Bay, northward along the Norwegian coast, and westward via from the Kola peninsula. These different migration routes would have met in northern Sweden.
Map showing the distribution of mitochondrial haplotypes, the locations of nuclear DNA sampling site, and origins of the phenotypic data used in the common greenhouse experiments in Pinus sylvestris populations, as well as exemplary dates (in calibrated years before present) of rising Pinus pollen curves (Donner et al. 1978; Seppä 1996; Vorren et al. 1996; Eronen et al. 1999; Eide et al. 2006). Possible migration routes of Scots pine are marked with arrows. The ice cap and water body represent the Ancylus lake situation approximately 10,000 years before present. In pie diagrams, the mitochondrial haplotype frequencies are indicated by white (type A, called AA in Naydenov et al. 2007) and black or striped (type C, called BA in Naydenov et al. 2007).
By looking at the dates of the first arrival of pine from pollen diagrams in Finland, Sweden, and Norway, it is evident that there is a difference of around 2450 years between its first appearance in southern Finland (Donner et al. 1978) and in northern Finland (Eronen et al. 1999) and of about 3170 years between its first appearance in southern Norway (Eide et al. 2006) and in the western coast of northern Norway (Vorren et al. 1996): distances of 860 and 1300 km, respectively. This would suggest a migration rate of around 0.4 km per year. This is a very rapid rate compared with recent estimates of migration rates in North American trees that acknowledge the existence of populations close to the ice (<100 m/year) (McLachlan et al. 2005; Petit et al. 2008). This is also somewhat faster than any migrations seen in the last 1000 years (Juntunen et al. 2006). The Scots pine calculations are based on the difference between just 2 pairs of pollen diagrams, but virtually all the available fossil evidence supports a rapid expansion of pine in the early postglacial. The rate of migration could have been enhanced by the different ecological conditions in the early postglacial because pines were colonizing a much more open landscape and, apart from the birch, there were no other tree species to compete with.
Genetic Traces of Colonization and Possible Admixture
Many temperate Central European tree populations show extensive genetic signals of admixtures between differentiated glacial refuge populations from the Iberian and Italian peninsulas and the Balkans (Petit et al. 2003). There have been fewer studies of Scandinavian populations, but in Norway spruce, there is some suggestion of a hybrid zone between admixture lineages in Central Sweden (Lagercrantz and Ryman 1990). Here we examine the genetic traces of admixture in northern Scots pine. Is the pattern of genetic variation in Nordic populations consistent with colonization from 2 different, differentiated sources? Or is it more likely that the current northern populations are derived from one source? Second, has recent selection eliminated any historical differences of adaptive differences between colonizing lineages?
An admixture between 2 genetically diverged sources of colonization should result in increased genetic variation at the admixture zone and increased linkage disequilibrium at least within closely linked nucleotide sites and in the early phases of admixture, between the cytoplasmic and nuclear genomes. If the differentiation between the 2 source populations is too low, the signs of admixture can be hard to find. In trees, which have a long juvenile phase, the probability of diverged lineages arising is lower than, for example, in annuals (Austerlitz et al. 2004). It is also important to evaluate the potential influence of gene surfing—increased genetic differentiation during a rapid expansion (Excoffier and Ray 2008). Such effects have been detected in North American Populus balsamifera (Keller et al. 2010). Colonization from one source could not only give rise to reduced diversity, as seen in many other species (Eckert et al. 2008; Muller et al. 2008; Pujol and Pannell 2008), but also increase linkage disequilibrium because of sequential bottlenecks (Hein et al. 2005).
Results from studies on mitochondrial DNA (mtDNA) variation (Sinclair et al. 1999; Naydenov et al. 2007; Pyhäjärvi et al. 2008) are consistent with each other and the dual colonization model, as the western and eastern sides of Scandinavia show different patterns (Figure 2). Unfortunately, the current level of available mtDNA polymorphism is not very informative, and the paternally inherited chloroplastDNA of conifers is not useful in this context due to low differentiation (Ennos 1994).
The northern populations (indicated in Figure 2) show rather high divergence at candidate loci for climatic adaptation (Table 1). The Russian Usinsk population is more highly diverged from the southern Swedish (Norra Gullabo) population (average FST = 0.069, 4 of 10 individual loci significantly different from zero) than European populations in general, consistent with the idea of somewhat diverged glacial populations. The northern Finnish Kolari population is intermediate, slightly closer to the Russian population. The Kolari population has the highest nucleotide and haplotypic diversity at these 10 loci, but the small differences between populations are not statistically significant (Table 2). Thus, there is not only little signal of increased diversity due to admixture but also no sign of reduced diversity due to sequential bottlenecks. Further, these northern populations also have roughly similar levels of variability as the central European populations of P. sylvestris (Pyhäjärvi et al. 2007). Linkage disequilibrium based on fairly long gene sequences seems to be consistently high even within the 2 colonizing lineages (Norra Gullabo, Sweden, and Usinsk, Russia, data not shown). Thus, we cannot detect increased linkage disequilibrium in the potentially admixed population. The within-gene estimates are not likely to be informative in this case for conclusions on admixture—we would need to have nucleotide sites that are further apart from each other than the length of the genes examined here.
Pairwise FST values between 3 populations of Pinus sylvestris averaged over 10 loci
| Norra Gullabo, Sweden | Kolari, Finland | |
| Kolari, Finland | 0.027 | — |
| Usinsk, Russia | 0.069 | 0.011 |
| Norra Gullabo, Sweden | Kolari, Finland | |
| Kolari, Finland | 0.027 | — |
| Usinsk, Russia | 0.069 | 0.011 |
Pairwise FST values between 3 populations of Pinus sylvestris averaged over 10 loci
| Norra Gullabo, Sweden | Kolari, Finland | |
| Kolari, Finland | 0.027 | — |
| Usinsk, Russia | 0.069 | 0.011 |
| Norra Gullabo, Sweden | Kolari, Finland | |
| Kolari, Finland | 0.027 | — |
| Usinsk, Russia | 0.069 | 0.011 |
Nucleotide diversity estimates (p) in 3 populations of Pinus sylvestris
| θWa | Hdb | |
| Kolari, Finland | 0.0045 (0.0036–0.0059) | 0.9012 |
| Norra Gullabo, Sweden | 0.0039 (0.0029–0.0054) | 0.8928 |
| Usinsk, Russia | 0.0037 (0.0028–0.0052) | 0.8536 |
| θWa | Hdb | |
| Kolari, Finland | 0.0045 (0.0036–0.0059) | 0.9012 |
| Norra Gullabo, Sweden | 0.0039 (0.0029–0.0054) | 0.8928 |
| Usinsk, Russia | 0.0037 (0.0028–0.0052) | 0.8536 |
Estimates are based on 10 loci.
Maximum posterior estimate for multilocus θW from silent sites; 95% credibility intervals in parentheses.
Haplotype diversity averaged over 10 loci.
Nucleotide diversity estimates (p) in 3 populations of Pinus sylvestris
| θWa | Hdb | |
| Kolari, Finland | 0.0045 (0.0036–0.0059) | 0.9012 |
| Norra Gullabo, Sweden | 0.0039 (0.0029–0.0054) | 0.8928 |
| Usinsk, Russia | 0.0037 (0.0028–0.0052) | 0.8536 |
| θWa | Hdb | |
| Kolari, Finland | 0.0045 (0.0036–0.0059) | 0.9012 |
| Norra Gullabo, Sweden | 0.0039 (0.0029–0.0054) | 0.8928 |
| Usinsk, Russia | 0.0037 (0.0028–0.0052) | 0.8536 |
Estimates are based on 10 loci.
Maximum posterior estimate for multilocus θW from silent sites; 95% credibility intervals in parentheses.
Haplotype diversity averaged over 10 loci.
We also examined whether the quantitative genetic variation shows any signs of admixture. Common garden studies of timing of budset were related to the mitochondrial DNA type of the maternal family (Table 3). This comparison shows that in the far northern population, close to the potential admixture zone, there is no association between the mtDNA variation and the adaptive variation. An association is seen only in the southern Swedish population, which should not be due to recent admixture. Thus, if the timing of budset was diverged between 2 colonizing lineages, natural selection for local adaptation has already eliminated such divergence. A similar finding has been made for quantitative variation in admixture zones of oak species (Kremer et al. 2002), but in oaks, the different maternal marker lineages still differed in nuclear marker frequencies.
Association between mitotypes and timing of budset in northern populations of Pinus sylvestris
| Population | Mitotype average | |||
| A | C | t | P | |
| Kolari, Finland | 89.40 | 91.22 | −1.54 | 0.14 |
| Uusikaupunki, Finland | 103.20 | 104.01 | −0.78 | 0.44 |
| Norra Gullabo, Sweden | 108.03 | 113.50 | −4.83 | <0.001 |
| Population | Mitotype average | |||
| A | C | t | P | |
| Kolari, Finland | 89.40 | 91.22 | −1.54 | 0.14 |
| Uusikaupunki, Finland | 103.20 | 104.01 | −0.78 | 0.44 |
| Norra Gullabo, Sweden | 108.03 | 113.50 | −4.83 | <0.001 |
Average timing of budset is expressed in days from sowing. The phenotypic data are from a greenhouse experiment conducted in 2003 and thus different from the data in Figure 1.
Association between mitotypes and timing of budset in northern populations of Pinus sylvestris
| Population | Mitotype average | |||
| A | C | t | P | |
| Kolari, Finland | 89.40 | 91.22 | −1.54 | 0.14 |
| Uusikaupunki, Finland | 103.20 | 104.01 | −0.78 | 0.44 |
| Norra Gullabo, Sweden | 108.03 | 113.50 | −4.83 | <0.001 |
| Population | Mitotype average | |||
| A | C | t | P | |
| Kolari, Finland | 89.40 | 91.22 | −1.54 | 0.14 |
| Uusikaupunki, Finland | 103.20 | 104.01 | −0.78 | 0.44 |
| Norra Gullabo, Sweden | 108.03 | 113.50 | −4.83 | <0.001 |
Average timing of budset is expressed in days from sowing. The phenotypic data are from a greenhouse experiment conducted in 2003 and thus different from the data in Figure 1.
The data may be consistent with the admixture of 2 colonization lineages, but for the most part, the traces of the distinct lineages are not clearly visible in the nuclear genes. Instead, all the populations still retain a signal of a very ancient population bottleneck and subsequent expansion (Pyhäjärvi et al. 2007; Kujala ST, Savolainen O, in preparation) The most recent expansion after the ice age may also have involved a large population expansion, but so few generations have passed that the genome has not yet accumulated the singleton mutations characteristic of such expansions.
How Could Adaptation Be So Rapid? Migration of Existing Genotypes or Standing Genetic Variation?
As described above, the rapid colonization of northern areas was accompanied by the generation of long clines in many traits, as shown by common garden experiments.
Clines could in principle originate through migration of different genotypes, each following their climatic envelopes. Many tree species, especially as seedlings, use photoperiods as an environmental cue for cessation of growth (Dormling 1973; Oleksyn et al. 1998; Howe et al. 2003; Böhlenius et al. 2006). The current tree populations in northern areas may stop growth if days are shorter than 20 h. These genotypes cannot have existed at high frequencies in populations that could grow, survive, and reproduce in Central European conditions. Thus, the northern populations have new phenotypic (and genotypic) combinations of photoperiodic reactions and, for example, cold tolerance, which must have arisen during the colonization phase (see also Ingvarsson et al. 2008).
New genotypes and phenotypes may arise by recombination and/or by genetic changes at individual loci. The genes that govern the variation in timing of growth clines in trees are still poorly known, with few exceptions. phyB seems to be involved in governing variation in the timing of growth cessation in Populus tremula (Ingvarsson et al. 2006, 2008). This gene and many of the studied candidate genes have shown weak clines or weak phenotypic effects in many species (García-Gil et al. 2003; Heuertz et al. 2006), suggesting either that the responsible loci have not been detected, the effects are quite small, or that the phenotypic changes are due to correlated small changes at several loci along the cline (Latta et al. 1998; LeCorre and Kremer 2003).
Adaptation to new environmental conditions can act on either newly arising mutations or on variation that already existed in the ancestral population, that is, standing genetic variation. Selection on standing genetic variation could promote rapid adaptation because beneficial alleles are immediately available, and no waiting period for new mutations is needed. These alleles can exist in considerable frequencies thus reducing the average fixation time. Also, as these variants can be old, they might have been “pre-tested and approved” by selection in past environments (reviewed by Barrett and Schluter 2007; Hermisson and Pennings 2005). Variants that have been favorable as Scots pine and other tree species have colonized northern areas have most likely existed in the Central European populations at some low frequency, as there have been repeated expansions during alternating climate conditions (Müller et al. 2003; Cheddadi et al. 2005; De Carvalho et al. 2010). Thus, we can speculate that adaptation during colonization of northern areas could have been accelerated by selection acting on standing genetic variation. We also presume that recombination between loci has generated new combinations of alleles and phenotypes, which did not exist in glacial survival areas.
Possible Responses to Climate Warming: Phenotypic Plasticity
Tree responses to climate warming are first due to phenotypic plasticity. Some of these may be adaptive, others are not. In the north, survival is expected to increase (Rehfeldt et al. 2002; Reich and Oleksyn 2008) and, indeed, height growth has already increased in northern Finland (Pensa et al. 2006).
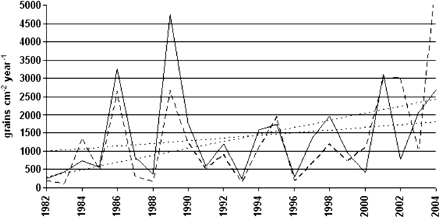
The warming climate has an influence not just on the individual trees but also on the reproductive traits of individual trees and hence on mating patterns of the populations. Until recently, seed and pollen production in the north have been on average very low and also highly variable between years (Koski and Tallqvist 1978; Savolainen 1996). During the last 10 years, the start of the thermal growing season has been 10 days earlier than the 1971–2000 average in the far north of Finland (Finnish Meteorological Institute). Concomitantly, male flowering of pines since the year 2000 has started 10 days earlier than the average of 1977–2007 (Heino S, personal communication). Pollen deposition records by pollen traps show that there is now also more abundant male flowering in northern Finland (Figure 3). Because of the earlier very limited pollen production, these changes may be more important in the north than in more southern populations that have produced much pollen also earlier.
Yearly deposition of Pinus pollen at 2 monitoring stations in northernmost Finland for the period 1982 to 2004. The amount of pollen reaching the ground is a good reflection of pollen production.
The changes in pollen and seed production can have important demographic consequences. In the warming climate, the pollen grains and seeds produced will have increased dispersal rates (Kuparinen et al. 2009). Seeds will also have increased chances of establishment. This has already contributed to range expansion observed at the northern limit of Scots pine (Juntunen et al. 2006) and altitudinal shifts in other northern species (Kullman 2002).
Possible Responses to Climate Warming: Evolution within Populations
Much of the literature on climate change response examines the potential of species to track their climatic envelopes, without considering genetic change. Many results have suggested that it will be very difficult to track the environment even with the increasing dispersal rates (Iverson et al. 2004; Kuparinen et al. 2009; Loarie et al. 2009).
In the long term, evolutionary changes will need to accompany range changes, as has also been the case during historical range expansion. How rapidly can trees evolve? To provide answers in a quantitative genetics framework, we have to make assumptions about the genetic basis of adaptive traits. The predictions of models can vary depending on the details of assumed traits (Hänninen 2006). It is very difficult to predict which traits will be most important for adaptation—for instance, new disease or insect resistance could be critical. From the past history, we know that adaptation to the length of the growing season has been important. As the average annual temperature is predicted to rise some 4 °C in the next century (Kattsov and Källen 2005), the sites at lat 68°N in Finland are predicted to experience growing season temperatures currently found in Helsinki (lat 60°N). We can then consider whether the pines of the Kolari population (lat 67.2°N) (see Figure 1) will evolve to have the mean timing of budset of Punkaharju (lat 61.8°N) in approximately 100 years? The populations are about 5 degrees of latitude apart and differ by 17 budset days in the experiment shown in Figure 1. This is more than 2 times the phenotypic standard deviation (SD) of the Kolari population.
When strong selection is applied in large experimental populations for specific traits, large initial responses can be obtained, until genetic variation is depleted or the deleterious side effects of selection prevent further advancement (Falconer and Mackay 1996). Introduced tree populations have also shown rapid phenotypic change, but the conditions in these experiments allow for very strong selection, as in a plant breeder's experiment rather than conditions of a natural population.
For a long-term response, theoretical considerations have suggested that populations could track environmental changes of a magnitude governed by the available genetic variation, the maximum growth rate (rmax) and the strength of stabilizing selection (σW). The critical rate of change of phenotypic optimum kc (relative to the phenotypic SD σP) is (Lynch and Lande 1993; Lynch 1996). If it is assumed that the maximum growth rate of a population is 0.5 and stabilizing selection is weak, then the rate is at most a few percentage of an SD per generation (Lynch 1996). Aitken et al. (2008) suggested that assuming weak selection but a high maximum growth rate, a much faster response would be possible (up to 40% of σP). Common garden–based estimates on Scots pine indicate very strong selection on timing of budset in Scots pine in the far north, such that even using the low reproductive capacity of 0.5, populations might be able to tolerate a faster changes in the phenotypic optimum (Knürr T, Kärkkäinen K, Savolainen O, in preparation). This could suggest rapid evolution, but the models may not be appropriate for such strong selection. Clearly many of the parameters governing responses are not known.
The above considerations ignored the influence of gene flow from outside the population. In the prevailing conditions, the lack of adaptation at range margins can be due to asymmetric gene flow from more central populations (García-Ramos and Kirkpatrick 1997). The current seeming lack of adaptation in the northernmost populations could well be due to asymmetric migration from the more southern populations. The female strobili become receptive before the males, and nonlocal pollen may be available for fertilization at this time in at least some years. The pollen shedding in southern Finland starts more than 3 weeks before that in northern Finland (Pessi and Pulkkinen 1994). In the north, some pine pollen is commonly recorded by aerobiological samplers before the local male flowering of pine begins (Ranta et al. 2008; Ranta and Pessi 2010). Varis et al. (2009) suggested that in at least some years this can result in pollination by southern trees. A similar window for pollination from outside the population has also been documented on an altitudinal scale, where lowland populations may pollinate receptive females higher on the slopes (Kärkkäinen 1991).
In the warming conditions, gene flow could aid adaptation. Gene flow was included in computer simulations on Scots pine and birch adaptation (Kuparinen et al. 2010). These data (based on detailed estimates of gene flow) showed, however, that the dispersal rate is not the most critical parameter for evolutionary response. This work also took into account the increased growth and survival of the northern Scots pine. The simulations showed that higher mortality would results in more evolutionary opportunity and a more rapid evolutionary change. Although the increased survival of Scots pine provides some buffering time, it also delays the evolutionary responses.
Until now, simulations on genetics have excluded many important genetic or evolutionary factors. The models have so far only included mortality due to frost or reduced growth. Genetic correlations with other adaptive traits may also influence the results (Etterson and Shaw 2001). The simulations have also ignored the role of the photoperiod (see below). And finally, as many tree species in the very north benefit from the warming climate, the fate of individual species may depend largely on the competition environment (Case et al. 2005).
Adaptation to Temperature and Photoperiod
Trees and other plants have adapted to local climate conditions by genetic differentiation with respect to their timing of growth cessation (Mikola 1982; Howe et al. 2003). Although adaptation is to the length of the thermal growing season (number of days with average daily temperature >5 °C), the environmental cue for growth cessation in many species from Drosophila to pitcher plant mosquitoes (Weiomyia smithii) and trees is frequently photoperiod (Bradshaw and Holzapfel 2001; Böhlenius et al. 2006). Photoperiod is important in seedlings, also in species with predetermined growth (Aitken et al. 2008). For instance, Mimura and Aitken (2010) recently provided empirical evidence that in Sitka spruce, photoperiodic control of the timing of budset is very important. Day lengths during the growing season change rapidly in high latitudes (see Figure 4). In this area, the climate differences between latitudes are large (e.g. in terms of length of thermal growing season). Day length is thus quite informative of the progress of the season. Populations from different latitudes have different critical photoperiods (at which growth ceases). Trees and other species are thus adapted to a combination of climate and the associated day length at each growing site.
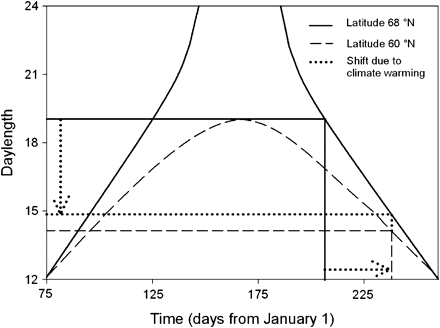
Relationship between the length of the growing season (governed by temperature) and photoperiod in northern latitudes. Day lengths along the season are shown for 2 different latitudes. The solid vertical and horizontal line show the correspondence between date of growth cessation and the day length at lat 68°N and the dashed vertical and horizontal lines show the same for lat 60°N. When the climate warms, the northern population should extend its growing season to cease growth at the current southern growth cessation date. However, this date translates to a different photoperiod at lat 68°N (15 h), not the same as lat 60°N (14 h). Note that the actual critical day lengths will be at some point before growth cessation, the figure is to illustrate this relationship. See text for explanation.
To be able to respond to climate change, populations need to evolve to have a new critical photoperiod, suited to their new environmental conditions. Figure 4 shows these relationships. Two curves show the day length at 2 different latitudes, one in southern Finland (60°N) and the other in northern Finland (68°N) during the growing season (Forsythe et al. 1995). In this example, the current northern Finnish populations’ (lat 68°N) growth cessation could be at day 203 (late July), corresponding to a day length of 19 h (on the y axis). As the climate warms (by about 4 °C), the thermal growing season will extend at the northern site (lat 68°N). The appropriate cessation of growth might be at day 230 (late August), which is the current growth cessation day of a southern (lat 60°N) population. In the south, this corresponds to a day length of about 14 h. The northern population will experience the new temperatures in its current photoperiodic environment. To achieve the appropriate growth cessation date, the northern population must evolve to stop growth at a date corresponding to 15 h, not at the date corresponding to 14 h. Thus, in the case of the warming climate, the required response is less on the photoperiod axis than on the calendar days (or temperature sum axis). This should facilitate adaptation—the appropriate photoperiodic reactions might be available in populations north of lat 60°N, perhaps at lat 62°N. Note that the critical day lengths are not those at the actual time of cessation of growth but much earlier, closer to the middle of the summer, when differences in day length between latitudes are even larger.
In W. smithii, the pitcher plant mosquito, a change in the critical photoperiod of the population has already been observed, a rare documented genetic change of local populations in response to climate warming (Bradshaw and Holzapfel 2001). Such changes have received very little attention but are expected in other populations as well.
In the very northernmost areas, day length as such in the middle of the summer may cease to be informative because the sun does not set at all. In those areas, the growth cessation reactions may be largely governed by light quality as the spectral composition varies between times of day, even if the days are 24 h long. Earlier work on Norway spruce (Picea abies; Ekberg et al. 1979) has documented differences between populations in sensitivity to the spectral quality of light.
Conclusions
The adaptation of pines and other economically important, often cultivated plants may be aided by carefully planned assisted migration, but other species will have to rely on the evolution within individual populations. Evolution of small and fragmented slowly growing populations may be limited by available genetic variability, and they may not be able to maintain the growth to sustainable response (Lynch 1996), even if there is an initial response to selection.
We have here concentrated the discussion on the northern, leading edge of the range of trees, especially with regards to Scots pine. Many of the conclusions will hold for other species with similar life histories in the north. For instance, Norway spruce and Sitka spruce (P. sitchensis; Mimura and Aitken 2007; Mimura and Aitken 2010) seem to share many of the characteristics of Scots pine. Still, differences in physiology between species may be important and give rise to different responses (Hänninen 2006). The evolutionary responses of species are expected to vary, as do the responses in distribution ranges: not all species are expanding their ranges northward or to higher altitudes (Parmesan 2006; Lenoir et al. 2008).
Many species in the north are experiencing increased survival, whereas the impacts of climate change are very different on the trailing edge, with populations of some species dying off. The high mortality in these populations may result in faster evolutionary responses, but the required genetic variation might not be available (Petit and Hampe 2005).
Possible responses of trees include phenotypic plasticity and adaptation. It is evident from the above considerations that these responses are not independent but that the phenotypic plasticity responses have an influence on demography and on the opportunity for selection. Migration, another possible response (Aitken et al. 2008), has been and will be accompanied by evolution within populations. Interspecific interactions (Kellomäki et al. 2001; Dullinger et al. 2004; Case et al. 2005) will also influence evolutionary changes in important ways.
Whether trees will have rapid evolutionary responses is still an open question. Some authors suggest that rapid evolutionary responses are possible (Berteaux et al. 2004; Hamrick 2004). So far, careful review has shown that there are few cases of convincingly demonstrated genetic changes in response to climate change (Gienapp et al. 2008), notably one well-documented case represents evolution of the photoperiodic response (Bradshaw and Holzapfel 2001). It is clear that in any species, many critical factors for predicting the responses are still unknown, such as the maximum reproductive rates and strength of selection or the role of interspecific competition. Further, predictions made on detailed understanding of genetic networks in different environmental conditions are so far only available for model organisms (Wilczek et al. 2010).
Funding
The Thule Institute of the University of Oulu; the National Population Genetics Graduate School; Biocenter Oulu; the EU Network of Excellence Evoltree (FP6 016322); the EU project Millennium (FP6 017008), the EU project Noveltree (FP7 211868).
We thank Torgny Persson for providing the Usinsk seeds and Folmer Bokma for help with Figure 4.
References
Author notes
Corresponding Editor: David Rand